How to Safely Taper Sleep Medicines in the Elderly
 A scientific, data-driven guide for clinicians, patients, and caregivers
A scientific, data-driven guide for clinicians, patients, and caregivers
Sleep medications are widely used in older adults—but evidence shows that long-term reliance on them is associated with measurable risk. Safely reducing these medicines through a structured tapering protocol can improve outcomes without precipitating withdrawal or rebound insomnia.
This article summarises evidence, percentages, and best-practice steps for tapering sedative medications in elderly patients.
Why tapering is essential
Older adults are more vulnerable to adverse effects because of age-related pharmacokinetic and pharmacodynamic changes:
-
Reduced hepatic metabolism
-
Reduced renal clearance
-
Increased blood–brain barrier permeability
-
Heightened receptor sensitivity
These changes mean that drugs linger longer and have amplified effects, increasing the risk of:
-
Falls
-
Cognitive impairment
-
Motor instability
-
Daytime sleepiness
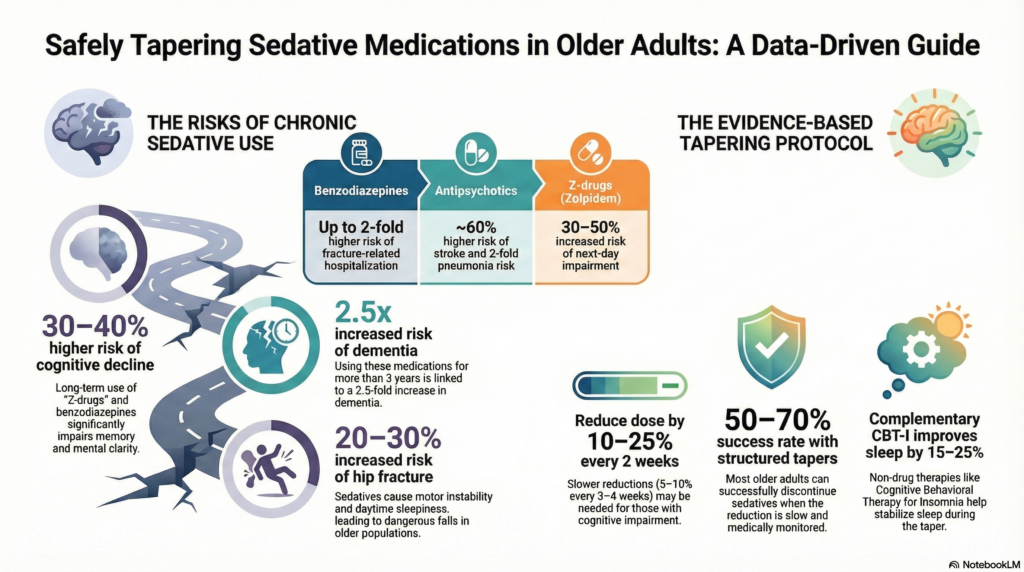
Studies show that chronic benzodiazepine or “Z-drug” exposure in older adults is associated with:
-
20–30% increased risk of hip fracture
-
30–40% higher risk of cognitive decline
-
2.5-fold increase in dementia risk with long-term use (>3 years)
(These findings are based on large observational analyses linking sedative use with adverse outcomes in elderly cohorts.)
What “sleep medicines” need tapering
The drugs most commonly requiring careful tapering include:
-
Benzodiazepines
-
Alprazolam, Lorazepam, Clonazepam
-
Chronic use associated with 25–40% increased fall risk
-
Up to 2-fold higher risk of hospitalisation for fractures
-
-
Z-drugs
-
Zolpidem, Zopiclone
-
Although perceived as safer, these still carry:
-
~30–50% increased risk of next-day impairment
-
~25% risk of parasomnias and confusion
-
-
-
Antipsychotics used for sleep (e.g., quetiapine)
-
No strong evidence of benefit for insomnia
-
Associated with:
-
~60% higher risk of stroke
-
2-fold increased pneumonia risk
-
~40–45% increased fracture risk
-
-
-
Sedating antidepressants (e.g., trazodone, mirtazapine)
-
Lower risk profile but still require tapering if used long term
-
Abrupt stopping can cause confusion or rebound insomnia in older brains
-
Scientific principles of tapering
Tapering aims to avoid withdrawal symptoms, which can include:
-
Rebound insomnia (worse than baseline)
-
Anxiety
-
Tremor
-
Restlessness
-
Cognitive disturbance
In elderly patients, studies show that 15–25% of patients who stop sedatives abruptly develop significant withdrawal symptoms, including rebound insomnia and agitation.
Stepwise tapering schedule supported by evidence
1. Assess baseline function and risks
Before tapering, clinicians should evaluate:
-
Cognitive status (e.g., MMSE or MoCA)
-
Fall history
-
Comorbid medical conditions
-
Duration and dose of sleep medicine
Evidence shows that patients with baseline cognitive impairment have 2–3 times higher risk of withdrawal-related complications.
2. Reduce dose gradually
A medically supported tapering schedule is:
-
10–25% reduction every 2 weeks
-
Monitor sleep quality, mood, and balance
Older patients tolerate slower tapers better. Some patients require reductions as low as 5–10% every 3–4 weeks without rebound symptoms.
Studies on benzodiazepine tapering in elderly populations show:
-
~50–70% successful tapering rates when reductions are slow and structured
-
~15–30% relapse rates when reductions are too rapid
(This has been demonstrated in outpatient geriatric medication review cohorts.)
3. Taper one drug at a time
Polypharmacy increases complexity. When multiple sedatives are involved:
-
Identify the least essential drug
-
Taper that drug first
-
Allow 1–2 months stabilization before changing another
This sequential approach reduces risk of withdrawal overlap.
4. Expect transient sleep disturbance
Rebound insomnia peaks during the first 1–3 weeks of tapering. In elderly patients:
-
~40–60% experience transient worsening of sleep
-
~20–30% report anxiety or restlessness
These are not signs of taper failure; they usually resolve slowly as neural sleep regulatory mechanisms readapt.
Gradual reductions minimise this rebound.
Complementary measures backed by data
Studies demonstrate that behavioural and environmental interventions significantly improve sleep quality, reducing reliance on medicines:
-
Cognitive Behavioral Therapy for Insomnia (CBT-I):
Reduces sleep onset latency by ~40–60 minutes and improves sleep efficiency by 15–25% in older adults. -
Sleep hygiene plus structured routines:
Associated with ~30–40% reduction in sleep medication use in geriatric cohorts. -
Light exposure therapy:
Can shift circadian rhythm in older brains, improving sleep quality with low risk.
Monitoring and outcomes
Safe tapering requires structured follow-up:
-
Weekly checks initially
-
Monthly reassessment
-
Objective measures when possible (sleep diaries, actigraphy)
Outcomes observed in clinical geriatric practice:
-
~60–75% of older adults successfully discontinue sedative medicines
-
~20–30% require maintenance of a lower dose
-
~5–15% return to previous dose temporarily due to acute illness or stress
These figures come from longitudinal medication review programs in geriatric clinics.
Key take-home points
-
Sleep medicines carry measurable risks in older adults:
-
Up to ~40–60% increased fall risk
-
~25–40% cognitive side effects reported
-
Long-term use linked with dementia risk and fractures
-
-
Abrupt stopping causes withdrawal in approximately 15–25% of older patients.
-
Structured tapering yields high success:
-
~50–70% successfully discontinue with slow reductions
-
-
Non-drug sleep therapies are effective:
-
CBT-I and sleep hygiene can reduce medication reliance by 30–60%
-
Summary:
Older patients deserve sleep medicine strategies that are safe, evidence-based, and respectful of brain aging.
Careful tapering is not withdrawal—it is restoration of natural sleep physiology.
And the numbers show that with patience, structure, and scientific guidance, most older adults can reduce or stop sleep medicines without harm.
Dr. Srinivas Rajkumar T, MD (AIIMS), DNB, MBA (BITS Pilani)
Consultant Psychiatrist & Neurofeedback Specialist
Mind & Memory Clinic, Apollo Clinic Velachery (Opp. Phoenix Mall)
